During Cellular Respiration, What Happens to the 6 Carbons in Glucose?
Identify the reactants and products of cellular respiration and where these reactions occur in a cell
At present that we've learned how autotrophs like plants convert sunlight to sugars, let's have a look at how all eukaryotes—which includes humans!—make use of those sugars.
In the process of photosynthesis, plants and other photosynthetic producers create glucose, which stores energy in its chemic bonds. And then, both plants and consumers, such as animals, undergo a series of metabolic pathways—collectively called cellular respiration. Cellular respiration extracts the free energy from the bonds in glucose and converts it into a form that all living things tin can employ.
Learning Objectives
- Describe the process of glycolysis and identify its reactants and products
- Describe the process of pyruvate oxidation and identify its reactants and products
- Describe the process of the citric acrid cycle (Krebs cycle) and identify its reactants and products
- Describe the respiratory concatenation (electron transport concatenation) and its role in cellular respiration
Cellular respiration is a process that all living things utilize to convert glucose into free energy. Autotrophs (like plants) produce glucose during photosynthesis. Heterotrophs (like humans) ingest other living things to obtain glucose. While the procedure can seem complex, this folio takes you lot through the central elements of each part of cellular respiration.
Glycolysis
Glycolysis is the first step in the breakdown of glucose to excerpt free energy for cellular metabolism. Nearly all living organisms carry out glycolysis as part of their metabolism. The procedure does not utilise oxygen and is therefore anaerobic (processes that use oxygen are called aerobic). Glycolysis takes place in the cytoplasm of both prokaryotic and eukaryotic cells. Glucose enters heterotrophic cells in two ways.
- Through secondary active transport in which the transport takes place confronting the glucose concentration gradient.
- Through a group of integral proteins called GLUT proteins, also known as glucose transporter proteins. These transporters assistance in the facilitated diffusion of glucose.
Glycolysis begins with the six carbon band-shaped structure of a unmarried glucose molecule and ends with two molecules of a iii-carbon carbohydrate chosenpyruvate(Effigy 1).

Figure one. Reactants and products of glycolysis.
Glycolysis consists of ten steps divided into two distinct halves. The first half of the glycolysis is also known as the energy-requiring steps. This pathway traps the glucose molecule in the cell and uses energy to change it and then that the six-carbon carbohydrate molecule tin can exist split evenly into the 2 three-carbon molecules. The 2nd one-half of glycolysis (too known equally the free energy-releasing steps) extracts energy from the molecules and stores information technology in the form of ATP and NADH, the reduced grade of NAD.
Beginning One-half of Glycolysis (Energy-Requiring Steps)

Figure two. The first half of glycolysis uses 2 ATP molecules in the phosphorylation of glucose, which is then separate into 2 three-carbon molecules.
Pace 1. The first step in glycolysis is catalyzed by hexokinase, an enzyme with broad specificity that catalyzes the phosphorylation of six-carbon sugars. Hexokinase phosphorylates glucose using ATP every bit the source of the phosphate, producing glucose-6-phosphate, a more reactive form of glucose. This reaction prevents the phosphorylated glucose molecule from continuing to interact with the GLUT proteins, and it can no longer leave the jail cell because the negatively charged phosphate will non permit it to cross the hydrophobic interior of the plasma membrane.
Step ii. In the second stride of glycolysis, an isomerase converts glucose-vi-phosphate into one of its isomers, fructose-6-phosphate. Anisomerase is an enzyme that catalyzes the conversion of a molecule into i of its isomers. This modify from phosphoglucose to phosphofructose allows the eventual separate of the sugar into two 3-carbon molecules.
Stride 3. The third stride is the phosphorylation of fructose-6-phosphate, catalyzed by the enzyme phosphofructokinase. A second ATP molecule donates a high-free energy phosphate to fructose-vi-phosphate, producing fructose-1,vi-bisphosphate. In this pathway, phosphofructokinase is a rate-limiting enzyme. It is active when the concentration of ADP is high; information technology is less active when ADP levels are low and the concentration of ATP is loftier. Thus, if at that place is "sufficient" ATP in the system, the pathway slows downwardly. This is a type of terminate production inhibition, since ATP is the end product of glucose catabolism.
Footstep iv. The newly added high-energy phosphates further destabilize fructose-1,6-bisphosphate. The fourth footstep in glycolysis employs an enzyme, aldolase, to cleave ane,vi-bisphosphate into two three-carbon isomers: dihydroxyacetone-phosphate and glyceraldehyde-3-phosphate.
Step 5. In the fifth footstep, an isomerase transforms the dihydroxyacetone-phosphate into its isomer, glyceraldehyde-iii-phosphate. Thus, the pathway volition go on with two molecules of a single isomer. At this point in the pathway, there is a net investment of energy from two ATP molecules in the breakdown of one glucose molecule.
Second Half of Glycolysis (Energy-Releasing Steps)
So far, glycolysis has cost the cell two ATP molecules and produced 2 small, three-carbon sugar molecules. Both of these molecules will go along through the 2nd half of the pathway, and sufficient energy will exist extracted to pay back the two ATP molecules used as an initial investment and produce a profit for the prison cell of two additional ATP molecules and two even higher-energy NADH molecules.

Figure 3. The second half of glycolysis involves phosphorylation without ATP investment (footstep six) and produces two NADH and iv ATP molecules per glucose.
Step 6. The sixth stride in glycolysis (Effigy 3) oxidizes the sugar (glyceraldehyde-3-phosphate), extracting high-energy electrons, which are picked up by the electron carrier NAD+, producing NADH. The sugar is then phosphorylated by the addition of a second phosphate grouping, producing one,iii-bisphosphoglycerate. Note that the second phosphate group does not require another ATP molecule.
Here once again is a potential limiting gene for this pathway. The continuation of the reaction depends upon the availability of the oxidized form of the electron carrier, NAD+. Thus, NADH must be continuously oxidized back into NAD+ in order to keep this step going. If NAD+ is not available, the second one-half of glycolysis slows down or stops. If oxygen is bachelor in the system, the NADH volition exist oxidized readily, though indirectly, and the loftier-energy electrons from the hydrogen released in this process will be used to produce ATP. In an environment without oxygen, an alternate pathway (fermentation) can provide the oxidation of NADH to NAD+.
Step 7. In the seventh footstep, catalyzed past phosphoglycerate kinase (an enzyme named for the reverse reaction), ane,3-bisphosphoglycerate donates a high-energy phosphate to ADP, forming one molecule of ATP. (This is an example of substrate-level phosphorylation.) A carbonyl group on the ane,three-bisphosphoglycerate is oxidized to a carboxyl group, and 3-phosphoglycerate is formed.
Step 8. In the eighth step, the remaining phosphate group in 3-phosphoglycerate moves from the third carbon to the second carbon, producing 2-phosphoglycerate (an isomer of 3-phosphoglycerate). The enzyme catalyzing this stride is a mutase (a type of isomerase).
Step 9. Enolase catalyzes the ninth stride. This enzyme causes 2-phosphoglycerate to lose water from its structure; this is a dehydration reaction, resulting in the germination of a double bond that increases the potential energy in the remaining phosphate bond and produces phosphoenolpyruvate (PEP).
Stride 10. The last step in glycolysis is catalyzed by the enzyme pyruvate kinase (the enzyme in this case is named for the opposite reaction of pyruvate's conversion into PEP) and results in the production of a 2d ATP molecule by substrate-level phosphorylation and the compound pyruvic acid (or its salt form, pyruvate). Many enzymes in enzymatic pathways are named for the reverse reactions, since the enzyme can catalyze both forward and reverse reactions.
Outcomes of Glycolysis
Glycolysis starts with glucose and ends with two pyruvate molecules, a total of 4 ATP molecules and two molecules of NADH. Two ATP molecules were used in the first half of the pathway to prepare the six-carbon ring for cleavage, and then the cell has a internet gain of two ATP molecules and 2 NADH molecules for its utilize.
If the cell cannot catabolize the pyruvate molecules further, it will harvest just two ATP molecules from one molecule of glucose. Mature mammalian cherry-red blood cells are not capable ofaerobic respiration—the procedure in which organisms convert energy in the presence of oxygen—and glycolysis is their sole source of ATP. If glycolysis is interrupted, these cells lose their ability to maintain their sodium-potassium pumps, and eventually, they dice.
The last pace in glycolysis volition not occur if pyruvate kinase, the enzyme that catalyzes the formation of pyruvate, is non available in sufficient quantities. In this situation, the entire glycolysis pathway will go along, but only ii ATP molecules will be fabricated in the 2nd one-half. Thus, pyruvate kinase is a rate-limiting enzyme for glycolysis.
In Summary: Glycolysis
Glycolysis is the start pathway used in the breakdown of glucose to excerpt free energy. It was probably one of the earliest metabolic pathways to evolve and is used by nearly all of the organisms on earth. Glycolysis consists of two parts: The first role prepares the half-dozen-carbon ring of glucose for cleavage into two three-carbon sugars. ATP is invested in the process during this one-half to energize the separation. The second one-half of glycolysis extracts ATP and high-energy electrons from hydrogen atoms and attaches them to NAD+. Two ATP molecules are invested in the offset half and four ATP molecules are formed past substrate phosphorylation during the second half. This produces a net gain of 2 ATP and 2 NADH molecules for the prison cell.
Figure 4 shows the unabridged procedure of glycolysis in one image:
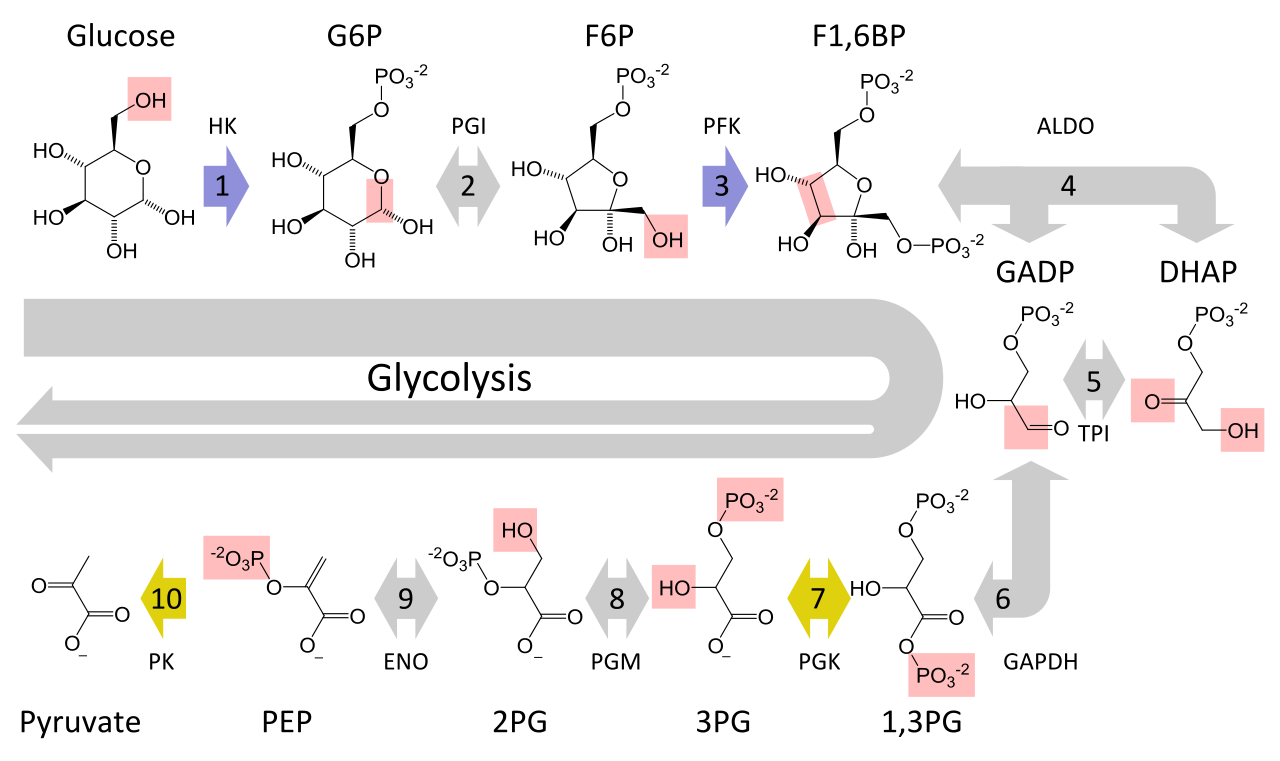
Effigy 4. Glycolysis
Pyruvate Oxidation
If oxygen is available, aerobic respiration volition go frontwards. In eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria, which are the sites of cellular respiration. There, pyruvate will exist transformed into an acetyl group that volition be picked upwardly and activated by a carrier chemical compound called coenzyme A (CoA). The resulting compound is called acetyl CoA. CoA is made from vitamin B5, pantothenic acrid. Acetyl CoA can be used in a variety of ways by the cell, just its major function is to evangelize the acetyl group derived from pyruvate to the next stage of the pathway in glucose catabolism.
Breakdown of Pyruvate
In order for pyruvate (which is the production of glycolysis) to enter the Citric Acrid Cycle (the next pathway in cellular respiration), it must undergo several changes. The conversion is a 3-step process (Figure v).

Figure v. Upon entering the mitochondrial matrix, a multi-enzyme circuitous converts pyruvate into acetyl CoA. In the process, carbon dioxide is released and one molecule of NADH is formed.
Stride i. A carboxyl group is removed from pyruvate, releasing a molecule of carbon dioxide into the surrounding medium. The result of this step is a two-carbon hydroxyethyl group bound to the enzyme (pyruvate dehydrogenase). This is the showtime of the six carbons from the original glucose molecule to be removed. This step gain twice (remember: in that location are ii pyruvate molecules produced at the cease of glycolysis) for every molecule of glucose metabolized; thus, 2 of the half dozen carbons will have been removed at the end of both steps.
Step 2. NAD+ is reduced to NADH. The hydroxyethyl group is oxidized to an acetyl group, and the electrons are picked upwards by NAD+, forming NADH. The loftier-free energy electrons from NADH volition exist used later to generate ATP.
Step 3. An acetyl group is transferred to conenzyme A, resulting in acetyl CoA. The enzyme-bound acetyl group is transferred to CoA, producing a molecule of acetyl CoA.
Note that during the second stage of glucose metabolism, whenever a carbon atom is removed, it is bound to ii oxygen atoms, producing carbon dioxide, ane of the major stop products of cellular respiration.
Acetyl CoA to COtwo
In the presence of oxygen, acetyl CoA delivers its acetyl group to a four-carbon molecule, oxaloacetate, to form citrate, a 6-carbon molecule with iii carboxyl groups; this pathway volition harvest the balance of the extractable energy from what began as a glucose molecule. This unmarried pathway is called past different names, but we will primarily call it the Citric Acid Wheel.
In Summary: Pyruvate Oxidation
In the presence of oxygen, pyruvate is transformed into an acetyl group attached to a carrier molecule of coenzyme A. The resulting acetyl CoA tin can enter several pathways, but most often, the acetyl group is delivered to the citric acid cycle for farther catabolism. During the conversion of pyruvate into the acetyl group, a molecule of carbon dioxide and 2 high-energy electrons are removed. The carbon dioxide accounts for 2 (conversion of two pyruvate molecules) of the six carbons of the original glucose molecule. The electrons are picked up by NAD+, and the NADH carries the electrons to a later pathway for ATP product. At this point, the glucose molecule that originally entered cellular respiration has been completely oxidized. Chemic potential energy stored within the glucose molecule has been transferred to electron carriers or has been used to synthesize a few ATPs.
Citric Acrid Bike
Like the conversion of pyruvate to acetyl CoA, the citric acid bicycle takes place in the matrix of mitochondria.This single pathway is called by unlike names: the citric acid bicycle (for the first intermediate formed—citric acid, or citrate—when acetate joins to the oxaloacetate), the TCA cycle (since citric acid or citrate and isocitrate are tricarboxylic acids), and the Krebs cycle, afterwards Hans Krebs, who first identified the steps in the pathway in the 1930s in dove flying muscles.
Well-nigh all of the enzymes of the citric acid cycle are soluble, with the single exception of the enzyme succinate dehydrogenase, which is embedded in the inner membrane of the mitochondrion. Different glycolysis, the citric acid cycle is a closed loop: The last part of the pathway regenerates the compound used in the first stride. The eight steps of the bike are a serial of redox, dehydration, hydration, and decarboxylation reactions that produce two carbon dioxide molecules, one GTP/ATP, and reduced forms of NADH and FADH2 (Figure 6). This is considered an aerobic pathway because the NADH and FADH2 produced must transfer their electrons to the next pathway in the system, which will use oxygen. If this transfer does non occur, the oxidation steps of the citric acid cycle besides exercise not occur. Note that the citric acid cycle produces very piffling ATP directly and does not straight consume oxygen.

Figure half dozen. In the citric acid wheel, the acetyl group from acetyl CoA is attached to a four-carbon oxaloacetate molecule to grade a six-carbon citrate molecule. Through a serial of steps, citrate is oxidized, releasing two carbon dioxide molecules for each acetyl group fed into the cycle. In the procedure, three NAD+ molecules are reduced to NADH, ane FAD molecule is reduced to FADH2, and one ATP or GTP (depending on the cell type) is produced (by substrate-level phosphorylation). Because the last product of the citric acid wheel is also the starting time reactant, the cycle runs continuously in the presence of sufficient reactants. (credit: modification of work by "Yikrazuul"/Wikimedia Commons)
Steps in the Citric Acid Wheel
Footstep 1. Prior to the starting time of the first pace, pyruvate oxidation must occur. So, the offset step of the bicycle begins: This is a condensation step, combining the two-carbon acetyl grouping with a four-carbon oxaloacetate molecule to form a six-carbon molecule of citrate. CoA is jump to a sulfhydryl group (-SH) and diffuses abroad to somewhen combine with some other acetyl group. This step is irreversible considering it is highly exergonic. The rate of this reaction is controlled by negative feedback and the amount of ATP available. If ATP levels increase, the rate of this reaction decreases. If ATP is in short supply, the charge per unit increases.
Stride 2. In stride two, citrate loses 1 water molecule and gains another as citrate is converted into its isomer, isocitrate.
Footstep 3. In footstep three, isocitrate is oxidized, producing a 5-carbon molecule, α-ketoglutarate, together with a molecule of CO2 and 2 electrons, which reduce NAD+ to NADH. This step is also regulated past negative feedback from ATP and NADH, and a positive effect of ADP.
Steps three and 4. Steps iii and four are both oxidation and decarboxylation steps, which release electrons that reduce NAD+ to NADH and release carboxyl groups that course CO2 molecules. α-Ketoglutarate is the production of step 3, and a succinyl group is the product of step iv. CoA binds the succinyl group to course succinyl CoA. The enzyme that catalyzes stride four is regulated by feedback inhibition of ATP, succinyl CoA, and NADH.
Step 5. In stride 5, a phosphate grouping is substituted for coenzyme A, and a high-free energy bail is formed. This energy is used in substrate-level phosphorylation (during the conversion of the succinyl group to succinate) to grade either guanine triphosphate (GTP) or ATP. There are ii forms of the enzyme, called isoenzymes, for this stride, depending upon the type of beast tissue in which they are found. One course is constitute in tissues that utilize large amounts of ATP, such as heart and skeletal musculus. This form produces ATP. The second form of the enzyme is establish in tissues that have a high number of anabolic pathways, such every bit liver. This form produces GTP. GTP is energetically equivalent to ATP; still, its use is more restricted. In particular, protein synthesis primarily uses GTP.
Step 6. Step vi is a dehydration process that converts succinate into fumarate. Two hydrogen atoms are transferred to FAD, producing FADH2. The energy independent in the electrons of these atoms is bereft to reduce NAD+ merely adequate to reduce FAD. Unlike NADH, this carrier remains attached to the enzyme and transfers the electrons to the electron transport chain straight. This process is fabricated possible past the localization of the enzyme catalyzing this pace within the inner membrane of the mitochondrion.
Pace vii. Water is added to fumarate during footstep seven, and malate is produced. The last stride in the citric acid cycle regenerates oxaloacetate past oxidizing malate. Another molecule of NADH is produced in the process.
Products of the Citric Acid Cycle
Two carbon atoms come into the citric acid cycle from each acetyl grouping, representing four out of the vi carbons of 1 glucose molecule. Ii carbon dioxide molecules are released on each turn of the cycle; however, these do not necessarily comprise the almost recently added carbon atoms. The two acetyl carbon atoms volition eventually be released on after turns of the cycle; thus, all six carbon atoms from the original glucose molecule are somewhen incorporated into carbon dioxide. Each plow of the cycle forms three NADH molecules and i FADH2 molecule. These carriers will connect with the final portion of aerobic respiration to produce ATP molecules. Ane GTP or ATP is too made in each wheel. Several of the intermediate compounds in the citric acid wheel tin be used in synthesizing non-essential amino acids; therefore, the wheel is amphibolic (both catabolic and anabolic).
In Summary: Citric Acrid Wheel
The citric acrid cycle is a series of redox and decarboxylation reactions that remove high-free energy electrons and carbon dioxide. The electrons temporarily stored in molecules of NADH and FADH2 are used to generate ATP in a subsequent pathway. One molecule of either GTP or ATP is produced by substrate-level phosphorylation on each turn of the cycle. There is no comparison of the cyclic pathway with a linear i.
Electron Transport Chain
You have just read virtually two pathways in cellular respiration—glycolysis and the citric acid cycle—that generate ATP. However, nearly of the ATP generated during the aerobic catabolism of glucose is non generated directly from these pathways. Rather, it is derived from a process that begins with moving electrons through a series of electron transporters that undergo redox reactions: the electron transport chain. This causes hydrogen ions to accumulate within the matrix space. Therefore, a concentration gradient forms in which hydrogen ions diffuse out of the matrix space by passing through ATP synthase. The current of hydrogen ions powers the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP.
Electron Send Concatenation
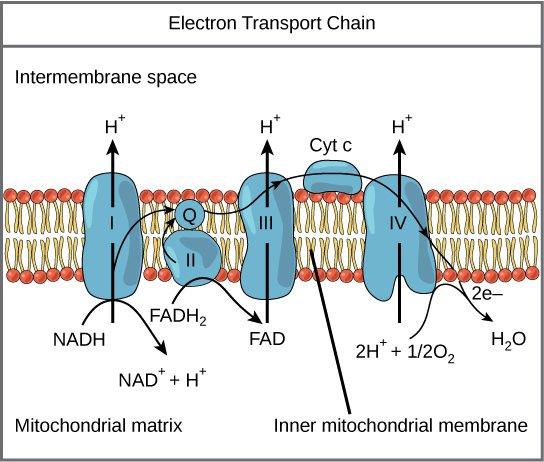
Figure 7. The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane infinite, and oxygen is reduced to grade h2o.
The electron ship chain (Figure 7) is the last component of aerobic respiration and is the only part of glucose metabolism that uses atmospheric oxygen. Oxygen continuously diffuses into plants; in animals, it enters the torso through the respiratory system. Electron ship is a series of redox reactions that resemble a relay race or bucket brigade in that electrons are passed apace from one component to the next, to the endpoint of the chain where the electrons reduce molecular oxygen, producing water. At that place are 4 complexes composed of proteins, labeled I through 4 in Figure 7, and the aggregation of these four complexes, together with associated mobile, accessory electron carriers, is called the electron transport chain. The electron transport concatenation is present in multiple copies in the inner mitochondrial membrane of eukaryotes and the plasma membrane of prokaryotes. Note, however, that the electron transport chain of prokaryotes may not require oxygen equally some live in anaerobic conditions. The common characteristic of all electron transport chains is the presence of a proton pump to create a proton gradient beyond a membrane.
Circuitous I
To start, two electrons are carried to the first circuitous aboard NADH. This circuitous, labeled I, is equanimous of flavin mononucleotide (FMN) and an fe-sulfur (Fe-Southward)-containing poly peptide. FMN, which is derived from vitamin B2, also called riboflavin, is i of several prosthetic groups or co-factors in the electron ship chain. Aprosthetic group is a non-protein molecule required for the action of a protein. Prosthetic groups are organic or inorganic, not-peptide molecules bound to a poly peptide that facilitate its role; prosthetic groups include co-enzymes, which are the prosthetic groups of enzymes. The enzyme in circuitous I is NADH dehydrogenase and is a very large poly peptide, containing 45 amino acrid chains. Complex I can pump four hydrogen ions beyond the membrane from the matrix into the intermembrane infinite, and it is in this way that the hydrogen ion gradient is established and maintained between the 2 compartments separated past the inner mitochondrial membrane.
Q and Complex II
Complex Ii straight receives FADH2, which does not pass through complex I. The compound connecting the showtime and second complexes to the tertiary isubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once information technology is reduced, (QH2), ubiquinone delivers its electrons to the adjacent complex in the electron send concatenation. Q receives the electrons derived from NADH from circuitous I and the electrons derived from FADH2 from complex 2, including succinate dehydrogenase. This enzyme and FADH2 form a small circuitous that delivers electrons directly to the electron transport chain, bypassing the first complex. Since these electrons featherbed and thus do non energize the proton pump in the first complex, fewer ATP molecules are made from the FADHtwo electrons. The number of ATP molecules ultimately obtained is direct proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex 3
The third complex is equanimous of cytochrome b, another Atomic number 26-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; this complex is as well chosen cytochrome oxidoreductase. Cytochrome proteins have a prosthetic grouping of heme. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. Every bit a consequence, the atomic number 26 ion at its core is reduced and oxidized as it passes the electrons, fluctuating between dissimilar oxidation states: Fe+ + (reduced) and Fe+ + + (oxidized). The heme molecules in the cytochromes have slightly dissimilar characteristics due to the effects of the unlike proteins binding them, giving slightly different characteristics to each circuitous. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the 4th complex of proteins and enzymes (cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c tin accept only one at a time).
Circuitous IV
The fourth complex is equanimous of cytochrome proteins c, a, and a3. This complex contains ii heme groups (one in each of the two cytochromes, a, and a3) and three copper ions (a pair of CuA and ane CuB in cytochrome a3). The cytochromes concord an oxygen molecule very tightly betwixt the fe and copper ions until the oxygen is completely reduced. The reduced oxygen then picks up two hydrogen ions from the surrounding medium to make water (H2O). The removal of the hydrogen ions from the organization contributes to the ion gradient used in the process of chemiosmosis.
Chemiosmosis
In chemiosmosis, the free free energy from the series of redox reactions just described is used to pump hydrogen ions (protons) across the membrane. The uneven distribution of H+ ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), owing to the hydrogen ions' positive charge and their aggregation on one side of the membrane.
If the membrane were open to diffusion by the hydrogen ions, the ions would tend to diffuse back across into the matrix, driven by their electrochemical gradient. Think that many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the help of ion channels. Similarly, hydrogen ions in the matrix space can only laissez passer through the inner mitochondrial membrane through an integral membrane protein called ATP synthase (Figure eight). This complex protein acts every bit a tiny generator, turned past the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of parts of this molecular motorcar facilitates the addition of a phosphate to ADP, forming ATP, using the potential energy of the hydrogen ion slope.
Practice Question
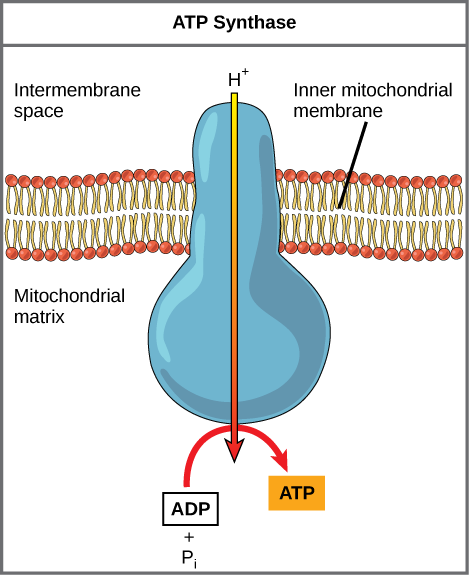
Figure 8. ATP synthase is a complex, molecular car that uses a proton (H+) gradient to form ATP from ADP and inorganic phosphate (Pi). (Credit: modification of work by Klaus Hoffmeier)
Dinitrophenol (DNP) is an uncoupler that makes the inner mitochondrial membrane leaky to protons. It was used until 1938 as a weight-loss drug. What outcome would you expect DNP to take on the change in pH across the inner mitochondrial membrane? Why do you think this might be an effective weight-loss drug?
Show Reply
After DNP poisoning, the electron transport concatenation tin no longer form a proton gradient, and ATP synthase tin can no longer brand ATP. DNP is an constructive diet drug because it uncouples ATP synthesis; in other words, subsequently taking it, a person obtains less energy out of the nutrient he or she eats. Interestingly, one of the worst side furnishings of this drug is hyperthermia, or overheating of the body. Since ATP cannot be formed, the free energy from electron transport is lost as heat.
Chemiosmosis (Figure nine) is used to generate xc percent of the ATP made during aerobic glucose catabolism; information technology is as well the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. Recall that the product of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation. The overall consequence of these reactions is the product of ATP from the free energy of the electrons removed from hydrogen atoms. These atoms were originally part of a glucose molecule. At the stop of the pathway, the electrons are used to reduce an oxygen molecule to oxygen ions. The actress electrons on the oxygen concenter hydrogen ions (protons) from the surrounding medium, and water is formed.
Practice Question
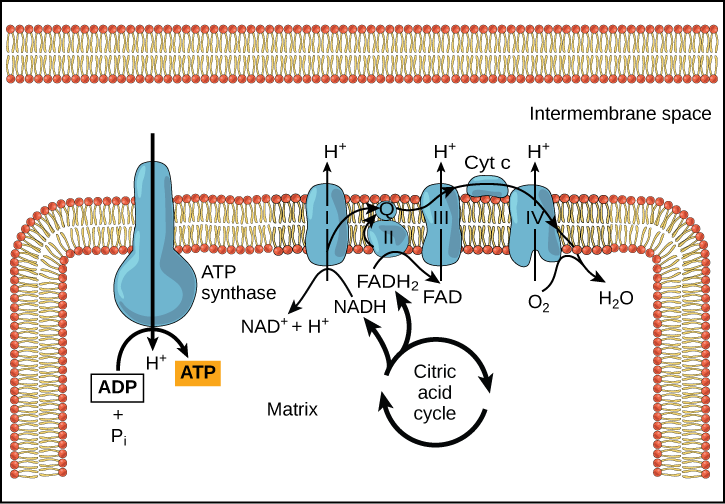
Figure 9. In oxidative phosphorylation, the pH gradient formed by the electron transport chain is used by ATP synthase to form ATP.
Cyanide inhibits cytochrome c oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you wait the pH of the intermembrane space to increase or subtract? What effect would cyanide have on ATP synthesis?
Prove Answer
Later on cyanide poisoning, the electron transport chain tin can no longer pump electrons into the intermembrane space. The pH of the intermembrane space would increase, the pH gradient would subtract, and ATP synthesis would stop.
ATP Yield
The number of ATP molecules generated from the catabolism of glucose varies. For example, the number of hydrogen ions that the electron send chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons beyond the membranes of the mitochondria. (The NADH generated from glycolysis cannot easily enter mitochondria.) Thus, electrons are picked up on the inside of mitochondria by either NAD+ or FAD+. As yous have learned before, these FAD+ molecules can transport fewer ions; consequently, fewer ATP molecules are generated when FAD+ acts as a carrier. NAD+ is used equally the electron transporter in the liver and FAD+ acts in the encephalon.
Another factor that affects the yield of ATP molecules generated from glucose is the fact that intermediate compounds in these pathways are used for other purposes. Glucose catabolism connects with the pathways that build or break down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For case, sugars other than glucose are fed into the glycolytic pathway for energy extraction. Moreover, the five-carbon sugars that course nucleic acids are made from intermediates in glycolysis. Certain nonessential amino acids can be made from intermediates of both glycolysis and the citric acrid bike. Lipids, such as cholesterol and triglycerides, are besides made from intermediates in these pathways, and both amino acids and triglycerides are cleaved down for energy through these pathways. Overall, in living systems, these pathways of glucose catabolism extract about 34 percent of the energy independent in glucose.
In Summary: Electron Ship Chain
The electron transport chain is the portion of aerobic respiration that uses gratis oxygen as the final electron acceptor of the electrons removed from the intermediate compounds in glucose catabolism. The electron transport chain is composed of 4 large, multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons betwixt them. The electrons are passed through a series of redox reactions, with a pocket-sized amount of free free energy used at three points to transport hydrogen ions across a membrane. This process contributes to the gradient used in chemiosmosis. The electrons passing through the electron transport concatenation gradually lose energy, Loftier-energy electrons donated to the concatenation by either NADH or FADH2 consummate the chain, every bit depression-free energy electrons reduce oxygen molecules and form h2o. The level of free energy of the electrons drops from about threescore kcal/mol in NADH or 45 kcal/mol in FADH2 to about 0 kcal/mol in water. The cease products of the electron transport chain are water and ATP. A number of intermediate compounds of the citric acid cycle can exist diverted into the anabolism of other biochemical molecules, such as nonessential amino acids, sugars, and lipids. These same molecules can serve as energy sources for the glucose pathways.
Let's Review
Cellular respiration is a collection of 3 unique metabolic pathways: glycolysis, the citric acid bicycle, and the electron transport chain. Glycolysis is an anaerobic procedure, while the other ii pathways are aerobic. In order to movement from glycolysis to the citric acid cycle, pyruvate molecules (the output of glycolysis) must exist oxidized in a process called pyruvate oxidation.
Glycolysis
Glycolysis is the first pathway in cellular respiration. This pathway is anaerobic and takes place in the cytoplasm of the cell. This pathway breaks downwards 1 glucose molecule and produces two pyruvate molecules. There are ii halves of glycolysis, with five steps in each half. The first one-half is known as the "energy requiring" steps. This half splits glucose, and uses upward ii ATP. If the concentration of pyruvate kinase is high enough, the 2nd one-half of glycolysis tin keep. In the second one-half, the "energy releasing: steps, 4 molecules of ATP and 2 NADH are released. Glycolysis has a net proceedsof 2 ATP molecules and 2 NADH.
Some cells (e.k., mature mammalian red claret cells) cannot undergo aerobic respiration, so glycolysis is their simply source of ATP. All the same, most cells undergo pyruvate oxidation and keep to the other pathways of cellular respiration.
Pyruvate Oxidation
In eukaryotes, pyruvate oxidation takes place in the mitochondria. Pyruvate oxidation can only happen if oxygen is bachelor. In this process, the pyruvate created by glycolysis is oxidized. In this oxidation process, a carboxyl group is removed from pyruvate, creating acetyl groups, which compound with coenzyme A (CoA) to class acetyl CoA. This process also releases COtwo.
Citric Acid Bicycle
The citric acid wheel (also known every bit the Krebs bike) is the second pathway in cellular respiration, and information technology also takes place in the mitochondria. The rate of the cycle is controlled by ATP concentration. When in that location is more ATP available, the rate slows down; when there is less ATP the charge per unit increases. This pathway is a closed loop: the terminal step produces the compound needed for the first footstep.
The citric acid cycle is considered an aerobic pathway because the NADH and FADH2 information technology produces act as temporary electron storage compounds, transferring their electrons to the adjacent pathway (electron send chain), which uses atmospheric oxygen. Each turn of the citric acid cycle provides a internet gain of CO2, ane GTP or ATP, and iii NADH and 1 FADH2.
Electron Send Chain
Most ATP from glucose is generated in the electron transport concatenation. It is the only part of cellular respiration that direct consumes oxygen; however, in some prokaryotes, this is an anaerobic pathway. In eukaryotes, this pathway takes identify in the inner mitochondrial membrane. In prokaryotes it occurs in the plasma membrane.
The electron send chain is made up of 4 proteins along the membrane and a proton pump. A cofactor shuttles electrons between proteins I–III. If NAD is depleted, skip I: FADHii starts on Two. In chemiosmosis, a proton pump takes hydrogens from inside mitochondria to the exterior; this spins the "motor" and the phosphate groups adhere to that. The move changes from ADP to ATP, creating 90% of ATP obtained from aerobic glucose catabolism.
Allow's Practice
At present that you've reviewed cellular respiration, this practice activeness will help you see how well you know cellular respiration:
Click here for a text-only version of the action.
Check Your Agreement
Reply the question(south) below to run into how well you understand the topics covered in the previous section. This short quiz doesnot count toward your class in the class, and yous tin retake information technology an unlimited number of times.
Use this quiz to check your understanding and decide whether to (one) study the previous section further or (2) movement on to the next section.
Source: https://courses.lumenlearning.com/suny-wmopen-biology1/chapter/cellular-respiration/
0 Response to "During Cellular Respiration, What Happens to the 6 Carbons in Glucose?"
Post a Comment